Hybridoma Generation for Therapeutic Antibody Discovery
Hybridoma technology and development is a critical step on the path towards therapeutic mAb discovery and antibody drug lead generation. Our partner GenScript's Hybridoma Generation for Therapeutic Antibody Discovery Service starts by selecting from a portfolio of immunization approaches to insure robust and appropriate immune responses. The combination of optimized immunization, fusion, and functional screening assays yields up to 100 high quality clonal hybridomas, assuring the success of your antibody drug discovery from the very beginning.
Key Features of Premium Hybridoma Development Services
1. One stop solution: from antigen production to hybridoma development and characterization.
2. Full spectrum of immunization approaches: protein, peptide, whole cells and DNA immunization.
3. High throughput screening
4. Comprehensive functional assay: Validated functional assay platforms provide reliable in vitro screening.
5. Readily integrated downstream services: Ab sequencing, Ab humanization, rAb production and anti-idiotype antibodies for your Ab drug PD/PK studies.
Premium Hybridoma Development Service Details (SC1693) (Service Flowchart)
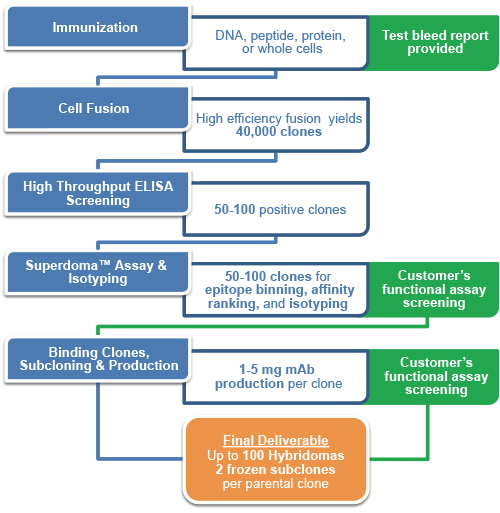
Premium hybridoma development service
| Phase | Description |
|---|---|
| Immunogen Preparation |
Protein immunogen supplied by GenScript:(Strongly Recommended)
Or qualified immunogen provided by customer:
|
| Immunization |
|
| Cell Fusion, Screening |
|
| Superdoma™ Assay and Isotyping |
|
| Small Scale Antibody Production |
|
| First Delivery |
|
| Functional Assay(Optional) |
|
| Subcloning, Expansion and Cryopreservation |
|
| Final Delivery |
|
| Large Scale Antibody Production and Storage Service(Optional) |
|
Note:
1) This protocol is for you reference, and we can customize your project based on your specific requirement.
2) We provide cell line storage service.

